Pharmacognostical and Pharmaceutical Evaluation of Rasnadi Basti in the Management of Vandhyatvo w.s.r to Anovulation
DOI:
https://doi.org/10.47070/ayushdhara.v10i4.1338Keywords:
Anovulation, Rasnadi basti, Pharmaceutical analysis, Pharmacognosy, VandhyatvaAbstract
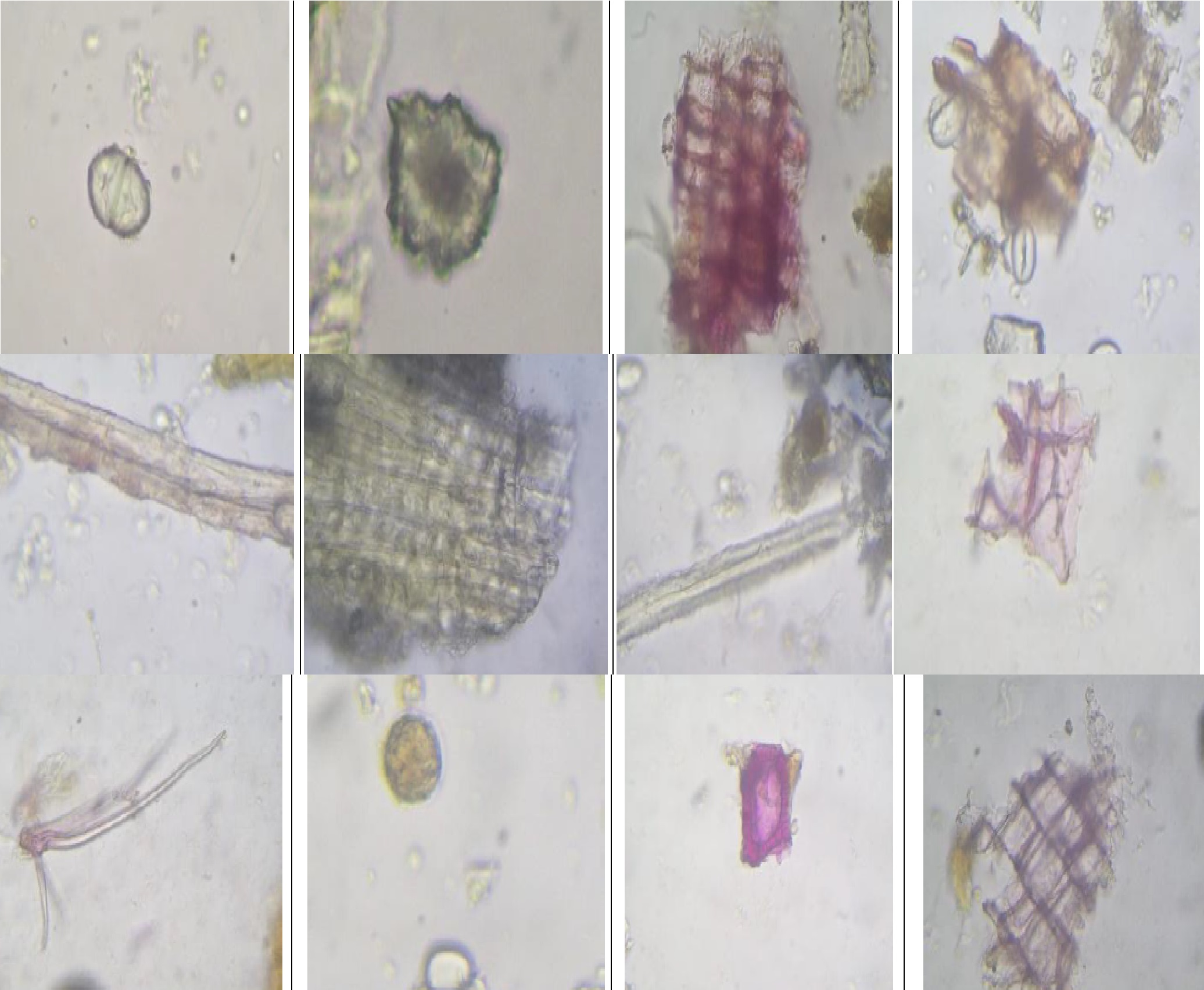
Rasnadi basti is mainly indicated for the Vataj disorders i.e., Vibandha, Anaha, Aruchi, Shoola, Vandhyatva, Pradara, Prameha, Yonidosha, Pandu, etc. It is having properties like Sarvaroganivarana, Shukrajanana, Vrushya, and Pumsavana Parama. Acharya Siddhinandan Mishra has mentioned the effectiveness of Rasnadi basti in the treatment of Vandhyatva. Vandhyatva (infertility) has got many etiological factors; one among them is anovulation. Vata is governing factor of the whole reproductive physiology & any vitiation in Vata may lead to anovulation. Basti Karma is the best choice for the treatment of Vata Dosha. In the study, Rasnadi Basti was given to one of the trial group having infertility due to anovulation. So, for the assurance of the quality of herbal compounds used to prepare Rasnadi basti, its pharmacognostic and pharmaceutical analysis was necessary. Methods: Freshly prepared Rasnadi Basti was subjected to microscopic evaluation for pharmacognostic & physiochemical analysis like specific gravity & solid content. Results: Pharmacognostical study showed the presence of certain identifying characteristics of the ingredients of Rasnadi basti i.e., Rasna, Eranda, Patala, Musta, Vacha, Pippli, Agnimantha, Kustha, Yastimadhu, etc. In pharmaceutical study, the preliminary physiochemical analysis showed solid content of 52.53% w/v & specific gravity of 1.0242. Conclusions: Pharmacognostical and physico-chemical observations revealed the specific characteristics of all active constituents of Rasnadi basti and confirmed the purity and genuinity of the formulation.
Downloads
Downloads
Published
Issue
Section
License
Copyright (c) 2023 AYUSHDHARA

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.


