Clinical Validation of Unani Pharmacopoeial Formulation Qurs-E-Dīdān in Dīdān Al-Am‘Ā’ (Intestinal Worms)
DOI:
https://doi.org/10.47070/ayushdhara.v8i6.856Keywords:
Dīdān al-Am‘ā’, Vermicidal, Vermifugal, TanninsAbstract
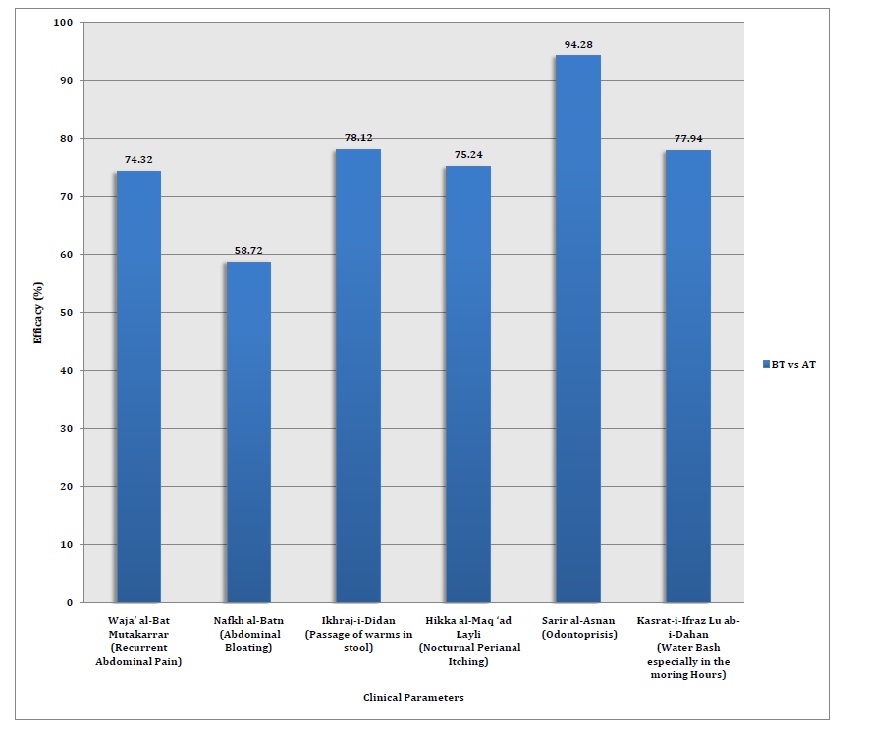
Qurs-e-Dīdān is an Unani Pharmacopeial formulation which is used since ancient period by Unani Physicians for the treatment of various intestinal worm infestation. The present study was carried out at Central Research Institute of Unani Medicine, Lucknow to scientifically validate the safety and efficacy of Unani Pharmacopeial formulation Qurs-e-Dīdān in Dīdān al-Am‘ā’ (Intestinal Worms) during the period 2015 to 2017. This was an open label, single arm, interventional study in which 68 subjects of either sex were randomly selected and given Qurs-e-Dīdān 250 mg. one tablet orally twice a day before meal for two weeks and the patients were observed weekly on the basis of subjective and objective clinical parameters which were recorded and the result was assessed on the basis of reduction in sign and symptoms on 4-point (0-3) scale and the presence or absence of ova, cyst or worms in the stool examination before and after the duration of protocol therapy and analyzed statistically using student’s paired 't’ test. The result was expressed as the Mean ± SD. The ova/cyst of worms passing in stools, recurrent abdominal pain, abdominal bloating, nocturnal perianal itching, odontoprisis, water brash were improved (P<0.01).
Downloads
Downloads
Published
Issue
Section
License
Copyright (c) 2022 AYUSHDHARA

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.


